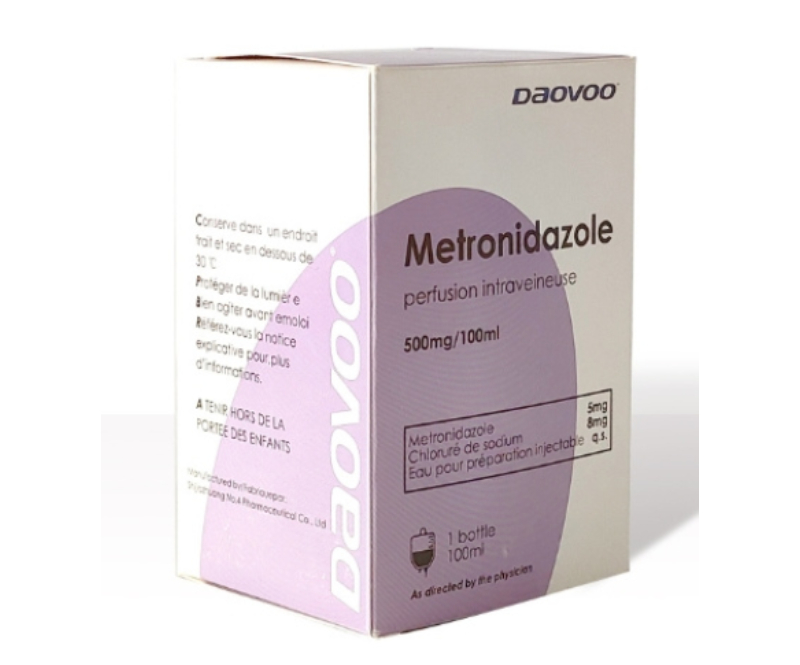
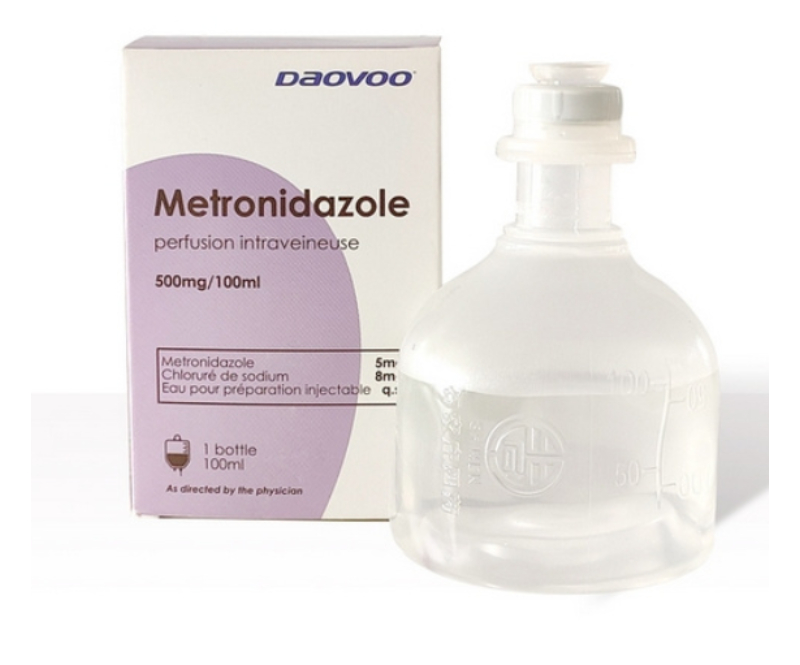
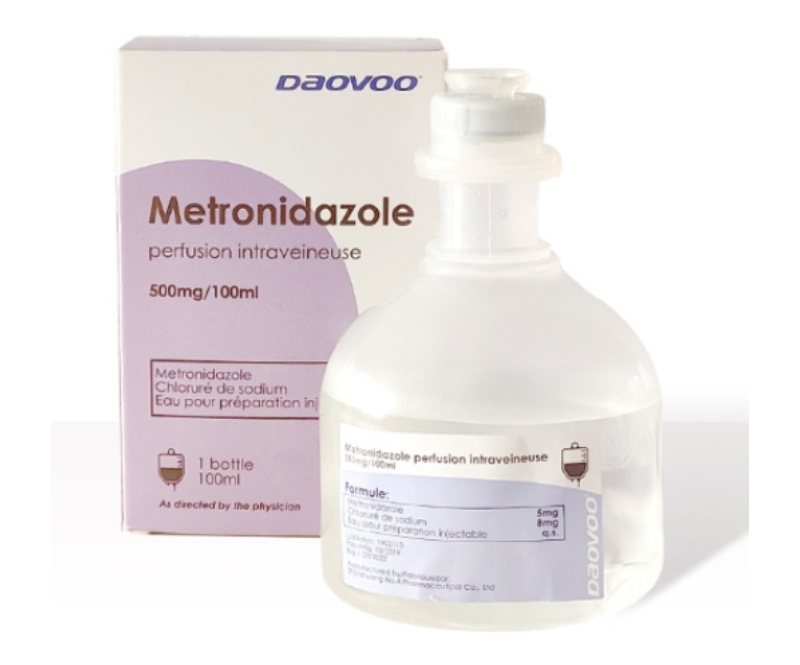
Metronidazole Infusion
Pharmacological category: Nitroimidazole antibiotics, anti-anaerobic bacteria & anti-protozoal drug
Mechanism of action: Penetrates into anaerobic bacteria and protozoan cells, reduces the nitro group to amino group, interferes with pathogenic microorganisms DNA synthesis and replication, exerts potent bactericidal and anti-protozoal effect. High sensitivity to most anaerobic Gram-positive/negative bacteria, Entamoeba histolytica, Trichomonas vaginalis and Giardia lamblia; no antibacterial effect on aerobic bacteria and facultative anaerobes.
Indications: Moderate to severe infections caused by sensitive anaerobic bacteria, including abdominal infection, pelvic infection, central nervous system infection, respiratory tract infection, skin and soft tissue infection, sepsis; intestinal/hepatic amebiasis, vaginal trichomoniasis, giardiasis; adjuvant therapy for mixed infections combined with other antibiotics.
Usage and dosage: Intravenous infusion only, avoid rapid intravenous injection. Adult: 0.5g once, 2 times a day, slow infusion for more than 30 minutes per dose; maximum daily dose 2.0g for severe infection. Pediatric: 7.5mg per kg body weight each time, 2~3 times a day, adjust dosage by age and condition, treatment course 7~14 days. Renal insufficiency patients no dosage adjustment needed, hepatic insufficiency patients reduce dosage properly.
Contraindications: Contraindicated in patients allergic to this product, nitroimidazole derivatives and any excipients; contraindicated in patients with active central nervous system diseases (epilepsy, encephalopathy) and hematological diseases; contraindicated in first-trimester pregnant women.
Cautious population: Use with caution in patients with severe hepatic insufficiency; use with caution in patients with peptic ulcer, gastritis and gastrointestinal bleeding history; second and third trimester pregnant women use cautiously after risk-benefit assessment; lactating women suspend breastfeeding during medication and within 24h after withdrawal; elderly patients use with reduced dosage; infants and young children use under strict medical guidance.
Adverse reactions: Common nausea, vomiting, abdominal distension, diarrhea, metallic taste in mouth and mild gastrointestinal discomfort; dizziness, headache, fatigue, insomnia and other central nervous symptoms; occasional rash, pruritus, leukopenia, thrombocytopenia and elevated transaminase; rare peripheral neuritis, numbness of limbs, convulsion and ataxia (more common in long-term/high-dose use); a small number of patients have phlebitis at infusion site, adverse reaction rate is slightly lower than injection formulation.
Notes: Infuse slowly (≥30min/100ml) to reduce vascular irritation and phlebitis; strictly prohibit drinking alcohol and alcoholic beverages during medication and within 7 days after withdrawal, prevent disulfiram reaction (flushing, headache, vomiting, palpitations, hypotension); do not mix with other drugs in the same infusion bottle; long-term medication monitor blood routine and liver function regularly; complete the full course of treatment to avoid anaerobic bacteria drug resistance.
Welcome to submit any questions and suggestions. We will reply to you as soon as possible. Thank you for your support and help.