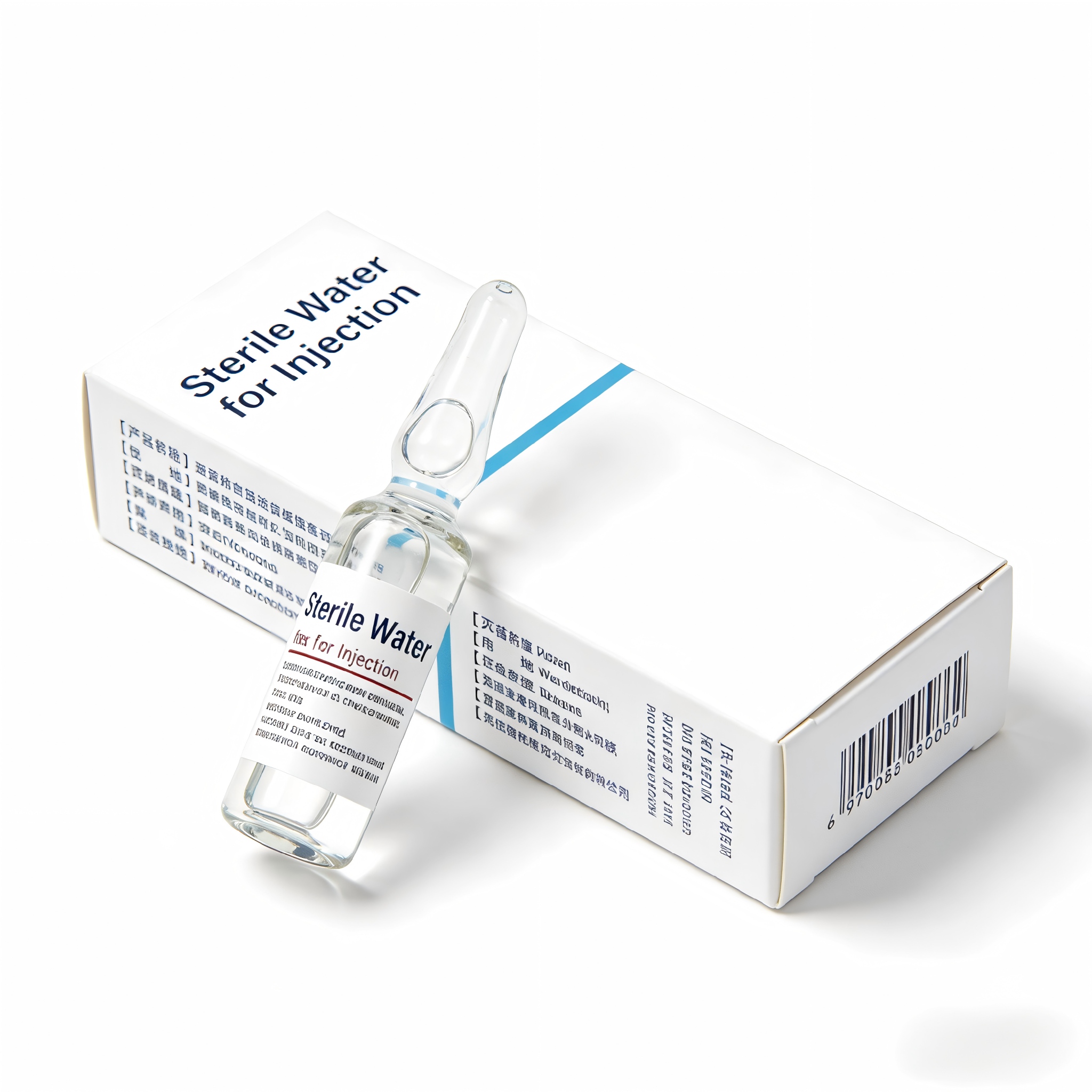
Sterile Water for Injection
Sterile Water for Injection is a highly purified water that meets stringent standards,it is primarily used for dissolving and diluting active pharmaceutical ingredients to prepare injectable solutions and powders for injection.
1. Dosage and Usage
The dosage and usage of Water for Injection depend on the specific application and the requirements of the pharmaceutical preparation or medical procedure. Here are some general guidelines:
Preparation of Injectable Solutions: The volume of WFI used will depend on the concentration of the active ingredient and the desired final volume of the injectable solution.For example, if a drug requires a concentration of 10 mg/mL and the total volume needed is 100 mL, the amount of WFI required will be calculated based on the solubility and concentration requirements of the drug.
Cleaning Medical Devices: The amount of WFI used for cleaning will depend on the size and complexity of the medical device.Typically, sufficient WFI is used to ensure that all surfaces are thoroughly rinsed and any residues are removed. This may involve multiple rinses with WFI to ensure complete cleaning.
Other Medical Applications: In applications such as endoscopy or dialysis, the volume of WFI used will be determined by the specific protocol of the procedure.For example, in dialysis, the volume of WFI used to prepare the dialysis fluid will be based on the patient's requirements and the specifications of the dialysis machine.
It is important to note that WFI should always be used in accordance with the guidelines provided by the relevant regulatory authorities and the specific requirements of the pharmaceutical or medical procedure. Healthcare professionals and pharmacists should refer to the appropriate guidelines and protocols to ensure the correct usage of WFI.
2. Storage
Proper storage of Water for Injection is essential to maintain its quality and ensure its suitability for use in medical and pharmaceutical applications.
3.Sterile Conditions
WFI must be stored in a sterile environment to prevent microbial contamination. This typically involves using sterile containers and maintaining aseptic handling practices.
4.Temperature Control
WFI should be stored at a controlled temperature, usually between 20°C and 25°C, unless otherwise specified.Temperature control helps to maintain the stability and quality of the water.
5.Protection from Light
WFI should be protected from direct sunlight and stored in a dark place to prevent any potential degradation or contamination.
6.Sealed Containers
WFI should be stored in tightly sealed containers to prevent contamination from the environment. This helps to maintain the water's purity and prevent the ingress of microorganisms or other contaminants.
Welcome to submit any questions and suggestions. We will reply to you as soon as possible. Thank you for your support and help.