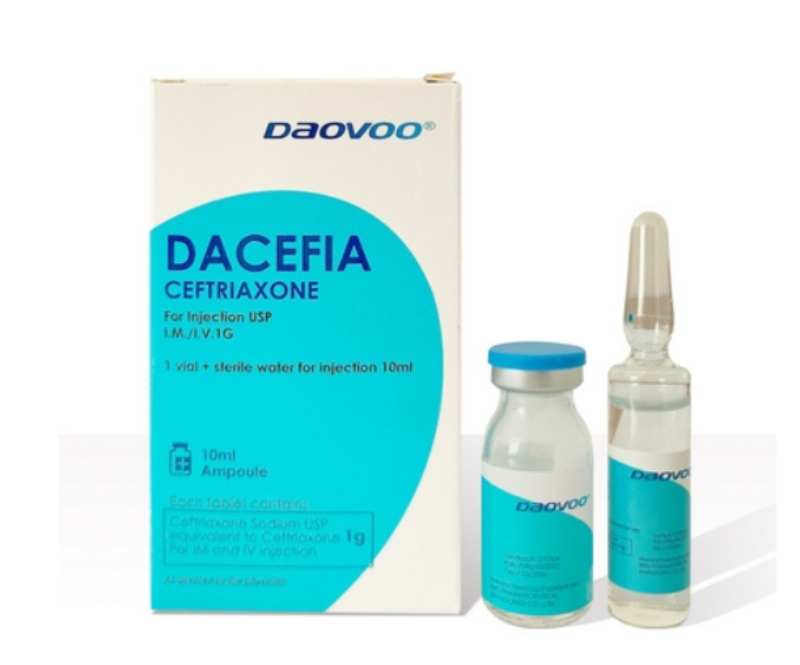
Ceftriaxone Sodium for Injection (1g)
Specification: 1g (powder vial) + matching Water for Injection
Pharmacological category: Third-generation cephalosporin antibiotics, broad-spectrum bactericidal drug
Mechanism of action: Inhibits bacterial cell wall peptidoglycan synthesis, destroys bacterial cell wall integrity and causes bacterial osmotic lysis and death, exerts potent broad-spectrum bactericidal effect. High sensitivity to most Gram-positive bacteria and Gram-negative bacteria, strong activity against drug-resistant Escherichia coli, Klebsiella pneumoniae, Salmonella, Shigella and Neisseria gonorrhoeae; stable to β-lactamase, long plasma half-life (8 hours), once-daily administration is sufficient, no cross-resistance with penicillin.
Indications: Moderate to severe infections caused by sensitive bacteria, including respiratory tract infection, urinary tract infection, biliary tract infection, abdominal cavity infection, pelvic infection, skin and soft tissue infection, sepsis, meningitis, osteomyelitis and septic arthritis; also effective for typhoid fever, paratyphoid fever and gonococcal infection; adjuvant treatment for mixed anaerobic infection combined with metronidazole, first-line antibiotic for severe bacterial infection in adults and children.
Usage and dosage: For intramuscular injection / intravenous drip only, reconstitute the powder with the matching Water for Injection first, then further dilute with 0.9% Normal Saline or 5% Glucose Injection for infusion. Adult: 1g once, once a day, intravenous drip over 30 minutes; increase to 1g twice a day (total 2g) for severe infection, maximum daily dose 4g. Pediatric: 20~80mg per kg body weight per day, once a day; neonates & infants: 20~50mg/kg/day, single daily dose, strictly follow doctor’s advice. General course of treatment: 7~14 days.
Contraindications: Contraindicated in patients allergic to this product, cephalosporin antibiotics and any excipients; contraindicated in patients with a history of severe immediate-type penicillin anaphylaxis (anaphylactic shock).
Cautious population: Use with caution in patients with severe hepatic and renal insufficiency (adjust dosage properly, monitor liver/renal function); use with caution in patients with gastrointestinal diseases (ulcerative colitis, pseudomembranous colitis); pregnant & lactating women use after risk-benefit assessment; elderly patients with organ hypofunction use with reduced dosage; neonates with hyperbilirubinemia use cautiously to avoid bilirubin encephalopathy; patients with coagulation dysfunction use with caution to reduce bleeding risk.
Adverse reactions: Mild and reversible adverse reactions, common mild nausea, vomiting, abdominal pain, diarrhea and anorexia; occasional skin rash, pruritus, urticaria (mild allergic reactions); rare elevated transaminase, thrombocytopenia, leukopenia, hemolytic anemia and hypoprothrombinemia; local pain at intramuscular injection site, phlebitis at intravenous infusion site (alleviated by slow drip); severe anaphylactic shock is rare (prevented by allergy test); long-term use may cause intestinal flora imbalance and secondary fungal infection. All adverse reactions resolve after drug withdrawal.
Notes: Mandatory skin allergy test before first administration, stop medication immediately and give emergency treatment if allergic reaction occurs; reconstitute strictly with the matching Water for Injection, do not mix with other antibiotics in the same infusion bottle; avoid mixing with calcium-containing solutions to prevent precipitation; complete the full course of treatment to avoid bacterial drug resistance; long-term medication monitor blood routine, liver/renal function and coagulation function regularly; intramuscular injection can be mixed with lidocaine to relieve local pain.
Welcome to submit any questions and suggestions. We will reply to you as soon as possible. Thank you for your support and help.