80+480mg Artemether and Lumefantrine Tablets
Pharmacological category: Antimalarial drug, compound artemisinin derivative preparation
Mechanism of action: Artemether and Lumefantrine exert a synergistic antimalarial effect. Artemether rapidly kills the erythrocytic stage of plasmodium by damaging the mitochondrial membrane structure of plasmodium; Lumefantrine inhibits the nucleic acid synthesis and metabolic process of plasmodium, prolongs the antimalarial effect and effectively reduces the risk of drug resistance. Oral absorption is significantly enhanced when taken with fatty food or milk, the plasma concentration reaches the peak in 4-8 hours, with long duration of action.
Indications: Used for the treatment of acute uncomplicated falciparum malaria in adults and children with body weight ≥35kg; also effective for vivax malaria, as the clinical first-line antimalarial medicine.
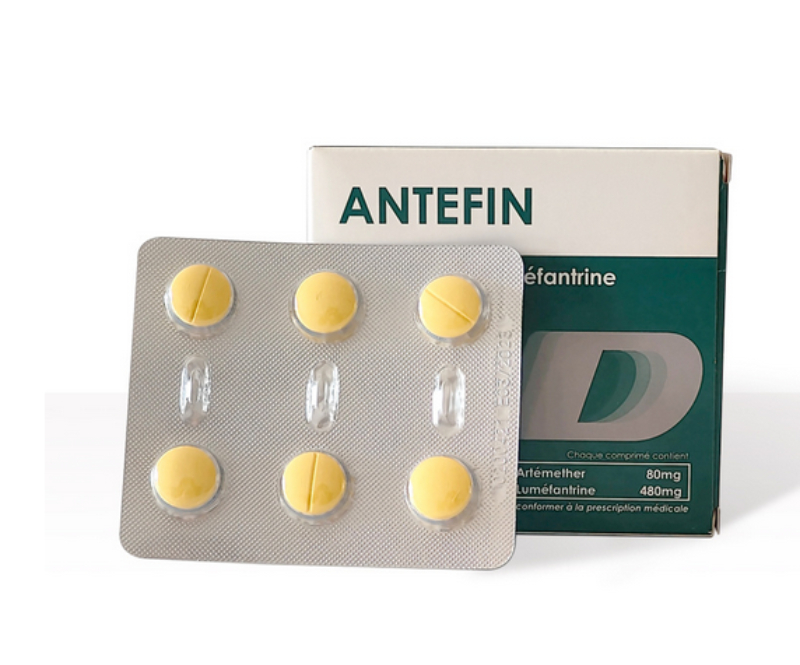
Usage and dosage: Oral administration, take with food, milk or fatty food to increase absorption. Adult and children ≥35kg: 1 tablet once, twice a day (morning and evening), for 3 consecutive days, a total of 6 tablets for the full course of treatment. Do not increase or decrease the dosage arbitrarily, ensure the complete course of treatment to avoid malaria recurrence and drug resistance.
Contraindications: Contraindicated in patients allergic to this product, artemether, lumefantrine and any excipients of the preparation.
Cautious population: Use with caution in patients with severe hepatic and renal insufficiency; use with caution in patients with cardiovascular diseases, QT interval prolongation, bradycardia and cardiomyopathy; pregnant and lactating women use with caution after weighing the therapeutic benefits and potential risks; elderly patients with weak physical condition and decreased organ function use with caution; children under 35kg are not recommended to use this specification, choose low-dose tablets instead.
Adverse reactions: Mild and reversible adverse reactions, common nausea, vomiting, abdominal pain, diarrhea, anorexia and other mild gastrointestinal discomfort; occasional dizziness, headache, fatigue, drowsiness and asthenia; rare skin rash, pruritus, joint pain and muscle pain; a small number of patients may have mild QT interval prolongation, which can recover spontaneously after drug withdrawal, no cinchonism reaction and no obvious liver and kidney toxicity.
Notes: This product is only for the treatment of acute malaria, not for malaria prevention and prophylaxis; it is only effective against the erythrocytic stage of plasmodium, and primaquine can be combined if necessary to eradicate gametocytes and prevent malaria transmission; avoid combined use with drugs that prolong the QT interval to reduce the risk of cardiac arrhythmia; swallow the tablet whole with warm water, do not chew or crush; store in a cool and dry place protected from light and high temperature.
Welcome to submit any questions and suggestions. We will reply to you as soon as possible. Thank you for your support and help.